医疗器材
Dedicated insights and know-how to advance your medical device.
Our specialized medical device division within our global CRO offers consultation and support, providing essential insights and expertise to help you maximize your device’s potential.
End-to-end support
Enable an optimized global regulatory strategy from preclinical to your product launch and beyond.
Focused experience
Access ~150 dedicated medical device consultants and the reach of our global organization.
ISO 13485 certified
Follow key ISO 13485 quality system requirements to simplify audits and reduce development burdens.
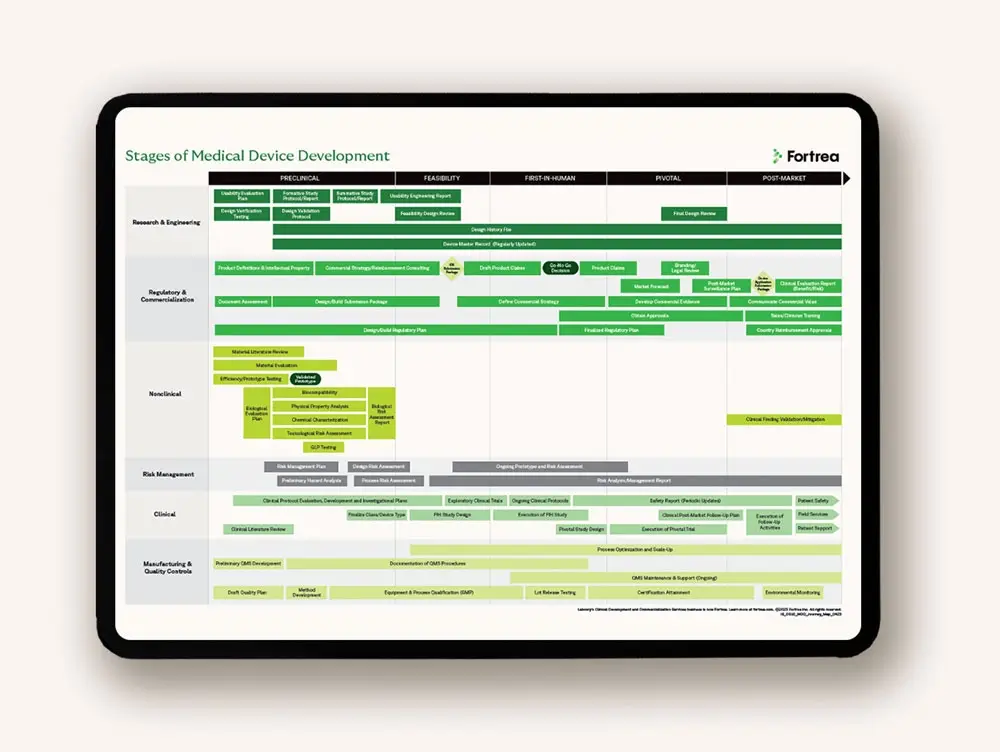
Specialized med device expertise—global CRO resources
Developing medical devices requires specialized skills and knowledge of specific global regulations, protocol design, quality systems and commercialization. Our global partnership provides the scale, strength and flexibility needed to advance with confidence.


Drive medical device development with global regulatory expertise
Innovative technology is only the beginning. You need a solid medical device development plan that can be adapted to produce compelling evidence and a convincing value proposition. As evidence is gathered, it informs and iterates regulatory, reimbursement, clinical and post-market strategies.
We examine every aspect of regulatory compliance to identify potential issues that could impact your design, materials, manufacturing methods or financial plans. Leveraging worldwide experience with regulatory agencies, we develop novel, targeted strategies to help you achieve regulatory approval in your target markets.
Enable efficiency across your medical device development
每项试验都有其独特之处。 Combined with optimized study execution, we will deliver the evidence you need for your submission while saving you time and money during the operational phase of your project.
Our advisors' deep experience can help you identify ways to improve efficiency and, where possible, reduce your timelines. They also work with you to pivot your strategy if unexpected findings occur, remaining flexible to meet unique study requirements and timelines. Let us partner with you from the beginning to improve efficiency and speed your time to market.